Search News
NEW YORK (CNNMoney.com) -- Drugmaker Johnson & Johnson said Tuesday that it has received a grand jury subpoena and is cooperating with a federal investigation stemming from multiple recalls of its popular non-prescription drugs.
The company, which also reported its quarterly results, said it was addressing a variety of legal actions, including lawsuits against its McNeil Consumer Healthcare unit, ongoing investigations into the recalls and a grand jury subpoena from the U.S. Attorney's Office for the Eastern District of Pennsylvania.
The company did not provide any further details about the subpoena.
Also on Tuesday, Johnson & Johnson cited the recalls in cutting its full-year profit outlook.
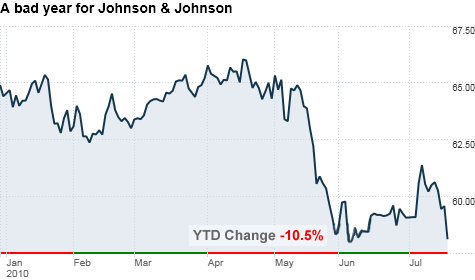
Johnson & Johnson (JNJ, Fortune 500) said it now expects to earn between $4.65 to $4.75 a share, excluding items, for the full year. The company had earlier issued full-year guidance of between $4.80 to $4.90 a share.
Analysts, on average, had expected the company to earn $4.80 a share for the year, according to Thomson Reuters.
The company's stock fell 2.5% in early trading.
The forecast reflects the impact of recalls announced earlier this year of Tylenol, Motrin, Benadryl and other medicines, as well as the suspension of a plant in Fort Washington, Pa.
In early May, McNeil recalled millions of children's Tylenol, Motrin and other over-the-counter medicines over quality concerns. Those products were made by McNeil, Johnson & Johnson's manufacturing division. The Fort Washington facility made all of the company's liquid pediatric drugs. (Read 'Tylenol plant: From bad to worse')
McNeil subsequently shut the plant for production after the Food and Drug Administration issued a scathing inspection report.
Last week, McNeil announced it was laying off 300 of more than 400 Fort Washington workers.
The company said it is taking steps to use other plants to make many of the products that it had manufactured in Fort Washington. But McNeil has also said that most of the products made in Fort Washington will not be available in stores before the end of the year.
Johnson & Johnson said the successive recalls, as well as the shutdown of the Fort Washington plant, resulted in a 27.5% sales drop of the company's over-the-counter drugs in the United States.
During a conference call with analysts Tuesday, Johnson & Johnson executives said the recalls and the shutdown of the Fort Washington plant will reduce annual sales of its over-the-counter products by about $600 million.
Meanwhile, another drugmaking facility operated by Johnson & Johnson -- this one in Lancaster, Pa., -- was flagged by the FDA last week over quality concerns. ![]()






| Index | Last | Change | % Change |
|---|---|---|---|
| Dow | 32,627.97 | -234.33 | -0.71% |
| Nasdaq | 13,215.24 | 99.07 | 0.76% |
| S&P 500 | 3,913.10 | -2.36 | -0.06% |
| Treasuries | 1.73 | 0.00 | 0.12% |
| Company | Price | Change | % Change |
|---|---|---|---|
| Ford Motor Co | 8.29 | 0.05 | 0.61% |
| Advanced Micro Devic... | 54.59 | 0.70 | 1.30% |
| Cisco Systems Inc | 47.49 | -2.44 | -4.89% |
| General Electric Co | 13.00 | -0.16 | -1.22% |
| Kraft Heinz Co | 27.84 | -2.20 | -7.32% |
|
Bankrupt toy retailer tells bankruptcy court it is looking at possibly reviving the Toys 'R' Us and Babies 'R' Us brands. More |
Land O'Lakes CEO Beth Ford charts her career path, from her first job to becoming the first openly gay CEO at a Fortune 500 company in an interview with CNN's Boss Files. More |
Honda and General Motors are creating a new generation of fully autonomous vehicles. More |
In 1998, Ntsiki Biyela won a scholarship to study wine making. Now she's about to launch her own brand. More |
Whether you hedge inflation or look for a return that outpaces inflation, here's how to prepare. More |