|
Glaxo merger scrutinized?
|
 |
June 27, 2000: 12:43 p.m. ET
Deal to create world's biggest drug company may face strict U.S. scrutiny
|
NEW YORK (CNNfn) - U.S. regulators are taking a close look at the proposed merger of Britain's Glaxo Wellcome PLC and SmithKline Beecham, potentially delaying approval of the deal, pharmaceutical industry analysts said Tuesday.
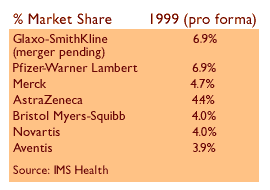
The $76 billion all-stock merger would create the world's largest drug company, edging out the new company formed by Pfizer Inc.'s (PFE: Research, Estimates) buyout of Warner-Lambert Co. in terms of revenue. The Pfizer deal closed earlier this month.
While European regulators have already approved the Glaxo-SmithKline pact with a few restrictions, the U.S. Federal Trade Commission is expected to require further conditions, analysts said.
The FTC is reviewing whether the combined company would control too great a share of the market for antidepressants, diabetes treatments and other drugs, they said.
"It is very clear that the FTC is the limiting factor right now," said Corey Davis, a pharmaceuticals analyst at Chase H&Q in New York. "They seem to have a much higher level of scrutiny than their European counterparts."
The FTC recently has signaled that it may get tougher on companies seeking to sell off some assets in order to assuage concerns over market domination. The commission's chairman, Robert Pitofsky, has said that even if a product is sold to a rival company, the buyer still might not have enough assets to provide genuine competition to the combined new company.
 "What makes this a much longer process in the U.S. is the FTC is looking not only at products on the market, but also ones under development," Davis said. "For one, those are very hard products to assess the value of ... and to decide if they're anticompetitive." "What makes this a much longer process in the U.S. is the FTC is looking not only at products on the market, but also ones under development," Davis said. "For one, those are very hard products to assess the value of ... and to decide if they're anticompetitive."
Davis and other analysts, however, say they have little doubt the merger ultimately will be approved.
"There may be some strict conditions on the merger, but nothing that's going to make anyone rethink the logic of putting these two companies together. It will be a great combination," Mark Ravera, an analyst at Mehta Partners in New York, told Reuters.
Companies stick to timetable
Glaxo (GLX: Research, Estimates) announced in January that it would buy the smaller SmithKline (SBH: Research, Estimates). Glaxo Chairman Richard Sykes initially forecast that the deal would receive regulatory approval in March, and both companies had thought the pact would close by late July. Analysts now say a September closing is more likely.
 The companies reiterated Tuesday that the closing's original timetable - sometime during the summer - remains unchanged. "The merger is on schedule. We have said we expect it to be completed this summer," said Jeremy Heynsfeld, a SmithKline spokesman. The companies reiterated Tuesday that the closing's original timetable - sometime during the summer - remains unchanged. "The merger is on schedule. We have said we expect it to be completed this summer," said Jeremy Heynsfeld, a SmithKline spokesman.
European regulators already ruled that SmithKline must divest its cancer-related nausea drug Kytril and herpes treatment Famvir because of product overlaps with Glaxo Wellcome. Sales of the two SmithKline drugs are projected to reach about $600 million this year.
But U.S. regulators could go further. One area of concern is in the diabetes market. Glaxo is developing a promising new compound, known as GI262570, which would compete against SmithKline's Avandia, a successful product already on the market.
If U.S. regulators force Glaxo to divest assets in this area, the company could be asked to license out development of the new compound to another firm. Or, the company could take the risky step of holding on to the promising compound - which has performed better in clinical trials than Avandia, Davis said.
"It's not clear to me what the resolution is going to be," he said.
 The companies also produce similar antidepressant drugs. The FTC could require the companies to divest Glaxo's Wellbutrin, which competes with SmithKline's Paxil. The companies also produce similar antidepressant drugs. The FTC could require the companies to divest Glaxo's Wellbutrin, which competes with SmithKline's Paxil.
In approving Pfizer's buyout of Warner-Lambert, U.S. regulators required that Warner-Lambert end its co-marketing agreement for sales of Celexa, an antidepressant that competes with Pfizer's Zoloft. The FTC also required several other divestitures before approving the deal.
Overall, the restrictions on the combined Glaxo-SmithKline are expected to total about $1.12 billion in lost sales, which is about 4.5 percent of estimated 2000 sales. Such divestitures would not have a significant impact on the bottom line, Davis said.
In Tuesday trading, American depositary receipts of Glaxo Wellcome gained 3/4 to 56-7/16, while SmithKline ADRs added 15/16 to 64-1/8. 
--from staff and wire reports
|
|
|
|
|
 |

|

