|
AHP warns about Norplant
|
 |
September 13, 2000: 5:23 p.m. ET
Drug maker offers to reimburse women for removal of contraceptive implant
|
NEW YORK (CNNfn) - American Home Products Corp. said Wednesday it could not guarantee the effectiveness of about 22,000 Norplant contraceptive implant kits shipped since October 1999, and also offered compensation to the affected patients who want the devices removed.
Wyeth-Ayerst Laboratories, a division of the Madison, N.J.-based drug maker, said seven lots of the product shipped after Oct. 20, 1999, may not release enough of the hormone levonorgestrel to block pregnancy. Norplant consists of six capsules inserted into a woman's arm that release hormones into the bloodstream on a time-release basis. The device is designed to be effective for up to five years.
The advisory follows a warning from the company last month that doctors should stop implanting Norplant devices from the seven lots in question because of questions over their effectiveness. Women who received Norplant implants prior to October 1999 are not affected, the company said.
The company advised women with implants from the potentially faulty lots to use backup, non-hormonal birth control and said it would provide $100 to each woman to compensate for the additional contraceptive costs. If patients with the affected Norplant contraceptives want the device removed, the company will pay the removal cost as well as reimburse the woman $700.
Health care providers will receive $350 for each patient who chooses to have the device removed as compensation for the removal procedure, pregnancy tests, office visit and administrative charges.
AHP spokesman Lowell Weiner said the company is not advising women on whether they should keep the devices inserted or not, saying the company does not yet have sufficient information yet on whether the affected implants are effective. AHP is continuing to test the lots, with additional information expected by the end of October.
"This is a private decision between the patient and her health care provider," he said.
The product accounted for $14 million of sales for AHP last year, less than 0.2 percent of the company's $9 billion in annual sales.
The warning is the latest trouble for AHP over the Norplant device. 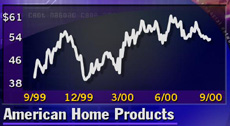 In August 1999, AHP agreed to pay about $50 million to more than 36,000 women to settle claims that the implants caused headaches, irregular menstrual bleeding, nausea and depression. In August 1999, AHP agreed to pay about $50 million to more than 36,000 women to settle claims that the implants caused headaches, irregular menstrual bleeding, nausea and depression.
Meanwhile, AHP also has entered a $3.75 billion federal settlement with former users of the fen-phen diet drug cocktail, which was pulled from the market after it was linked to heart valve problems. American Home recalled fenfluramine -- the "fen" part of the popular diet remedy -- as well as Redux, a chemical cousin, in 1997.
Shares of American Home (AHP: Research, Estimates) edged up $1.31, or about 2.5 percent, to $54.50 Wednesday. 
|
|
|
|
|
|
American Home Products
|
Note: Pages will open in a new browser window
External sites are not endorsed by CNNmoney
|
|
|
|
 |

|

