|
Europe OKs new Claritin
|
 |
January 16, 2001: 12:02 p.m. ET
European Commission clears updated version of Schering-Plough blockbuster
|
NEW YORK (CNNfn) - Schering-Plough Corp. said Tuesday that European regulators have approved a next-generation version of its blockbuster allergy drug Claritin, which is expected to lose its patent protection next year.
The European Commission approved the drug, a non-sedating antihistamine known as desloratadine, throughout the 15-country European Union.
However, the Kenilworth, N.J.-based drug maker still is awaiting word on regulatory approval of desloratadine from the U.S. Food and Drug Administration. The company hopes to bring the highly anticipated drug to the U.S. market by the beginning of the spring allergy season.
Click here to check the performance of drug stocks today
Schering-Plough is looking to the drug to help compensate for an expected drop-off in sales for Claritin once cheaper, generic versions are allowed to go on the market. Claritin had sales of roughly $2.7 billion in 1999, making it the world's No. 1 allergy treatment.
 "The approval of desloratadine in Europe represents a significant step in establishing desloratadine as an important new therapy for the treatment of seasonal allergies on a global basis," said Roch F. Doliveux, president of Schering-Plough International. "The approval of desloratadine in Europe represents a significant step in establishing desloratadine as an important new therapy for the treatment of seasonal allergies on a global basis," said Roch F. Doliveux, president of Schering-Plough International.
The drug will be marketed in Europe under the names Aerius and Neoclarityn.
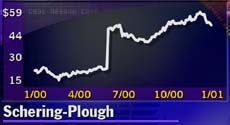
Schering-Plough (SGP: Research, Estimates) developed desloratadine in conjunction with biotechnology firm Sepracor Inc. (SEPR: Research, Estimates). In midday trading Tuesday, Schering-Plough shares rose $1.88 to $52.63, while stock in Marlborough, Mass.-based Sepracor added $1.19 to $72. 
|
|
|
|
|
|
Schering-Plough
|
Note: Pages will open in a new browser window
External sites are not endorsed by CNNmoney
|
|
|
|
 |

|

