|
Patients press for Enbrel
|
 |
February 22, 2001: 3:39 p.m. ET
Immunex created a blockbuster arthritis drug. But supply can't meet demand
By Staff Writer Martha Slud
|
NEW YORK (CNNfn) - Immunex Corp.'s rheumatoid arthritis treatment Enbrel is on its way to becoming a biotechnology blockbuster, racking up sales of about $650 million last year. But the company is having trouble keeping up with demand for the two-year-old treatment, a situation that experts say suggests a broader manufacturing problem in the growing biotech industry.
Seattle-based Immunex (IMNX: Research, Estimates), which is 41 percent owned by drugmaker American Home Products Corp. (AHP: Research, Estimates) of Madison, N.J., is rapidly trying to boost manufacturing capacity for Enbrel, a treatment that costs about $12,000 a year per patient. The company is retrofitting a plant in Rhode Island to manufacture the drug, which will double supply, but production is not expected to begin there until early next year.
Meanwhile, in a rare move, the drugmaker has asked people taking Enbrel to register with the company in an attempt to better manage demand for the medicine, while new patients are on a waiting list. About 70,000 patients are now on the drug, while another 1,000 are waiting for it.
Click here for more on biotech stocks
Enbrel, which is taken by injection, is made from a natural human protein that blocks high levels of a substance in the immune system known as tumor necrosis factor, or TNF, that is linked to inflammatory disease. Company researchers also are studying links between TNF and other diseases such as psoriasis and congestive heart failure.
| |

|
|
| |
|
|
| |
I think the possibility for an industrywide manufacturing bottleneck in the roughly two-year horizon is a real one.
|
|
| |
|
|
| |

|
|
| |
|
|
| |
Akhtar Samad
Biotech analyst
Bear Stearns |
|
"We will see that that is one of the biggest discoveries at least of the decade in medicine, because it is such a key mediator of diseases," said Peggy Phillips, Immunex's chief operating officer.
Enbrel received regulatory approval in November 1998 for treatment of rheumatoid arthritis, which affects about 2 million Americans. But producing the drug is a complex biological process that requires specialized manufacturing facilities that can take years to build. In addition, Enbrel requires a large amount of drug per dose – much more than other biotech products on the market.
The company notes that the number of U.S. patients taking Enbrel is roughly the same as the number taking Biogen Inc.'s (BGEN: Research, Estimates) multiple sclerosis treatment Avonex, but Biogen only needs about 110 grams of protein to produce a year's worth of Avonex, while Enbrel requires a weighty 182 kilograms for a year's supply.
Enbrel also uses up to 100 times more drug than Amgen Inc.'s (AMGN: Research, Estimates) Epogen and Neupogen, two other leading biotechnology drugs on the market, according to Immunex's figures.
The manufacturing squeeze led the company to revise downward its 2001 sales projections for Enbrel last month, triggering analyst downgrades and a stock sell-off. Despite strong demand from patients, Immunex said 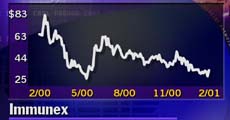 Enbrel sales should total only about $750 million this year, down from earlier forecasts of about $850 million. Enbrel accounts for about 80 percent of Immunex's total sales. Enbrel sales should total only about $750 million this year, down from earlier forecasts of about $850 million. Enbrel accounts for about 80 percent of Immunex's total sales.
Shares have climbed in recent days, though, as investors have taken the ongoing manufacturing crunch as a positive sign about demand for the drug. The stock is trading around $31.50 per share, but it is still about 62 percent down from its 52-week high of $83.60.
For Immunex, boosting its production capacity will be key to future success of Enbrel, said Akhtar Samad, a biotech analyst at Bear Stearns.
"The bottom line is that Enbrel is likely to have quite a high level of success in the trials that are ongoing, so there's a pressing need for Immunex to rapidly generate the requisite manufacturing capacity to leverage the success the company is having in the clinic," he said.
The overall manufacturing problem could be a major one as the biotech industry further matures, Samad said. Biotech experts foresee an explosion of new drug development through discoveries gleaned from the human genome project, but companies first will need to build the infrastructure to bring their products to market.
"I think the possibility for an industry wide manufacturing bottleneck in the roughly two-year horizon is a real one," he said. "Most of the leading players in the space are trying to be as proactive as possible to offset that risk by securing new manufacturing facilities for biologicals."
The production crunch also could give some of Immunex's competitors a window of opportunity. Johnson & Johnson (JNJ: Research, Estimates) makes arthritis treatment Remicade. Knoll Pharmaceuticals, a unit of Germany's BASF AG that is being acquired by Abbott Laboratories (ABT: Research, Estimates), is testing an arthritis drug, known by research number D2E7, that experts say could compete with Enbrel. 
|
|
|
|
|
 |

|

