NEW YORK (CNN/Money) - U.S. antitrust regulators are investigating the patent claims of three major drugmakers to see if they are making false claims to keep generic versions of their drugs from coming to market, according to a published report Tuesday.
The Federal Trade Commission is investigating patent claims of Bristol-Myers Squibb Co. for its anxiety drug BuSpar and its breast cancer drug Taxol, GlaxoSmithKline PLC for its Paxil antidepressant, and Biovail Corp. for heart drug Tiazac, according to a report in USA Today that cited unnamed sources familiar with the investigation.
"[The investigation is] something we've disclosed in our annual report," GlaxoSmithKline (GSK: up $0.21 to $43.42, Research, Estimates) representative Mary Anne Rhyne told CNN/Money. "We believe the patents are valid, we believe they're properly listed, and we're certainly cooperating with the investigation."
A Bristol-Myers Squibb spokesman told CNN/Money the company was complying with all FTC requests for information about Taxol and BuSpar.
"We licensed a patent that we filed ... because we believed and believe now that the patent is absolutely validly listed," Biovail (BVF: down $1.20 to $33.40, Research, Estimates) general counsel Kenneth Cancellara told CNN/Money. "We wouldn't have done it otherwise."
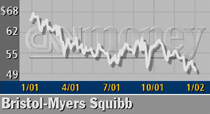
FTC spokesman Mitch Katz would only confirm an investigation into Bristol-Myers' (BMY: down $0.18 to $30.09, Research, Estimates) Taxol.
Katz said the FTC has also expressed interest in a case pending involving BuSpar's Orange Book listing and may want to file an amicus brief in that case.
Drugmakers have found their patent claims for some of their big-ticket drugs coming under fire, as competitors and other groups clamor for cheaper generic drugs.
Twenty-nine state attorneys general and several individuals already have sued Bristol-Myers Squibb in relation to its BuSpar patent, while Biovail and Andrx, two generic drug companies, are fighting over Tiazac.
The U.S. Food and Drug Administration lists new drug patents in its Orange Book without researching their validity, the report said. The drug companies are responsible for certifying that their patents are proper.

|

