|
Drug firms post 3Q gains
|
 |
October 19, 2000: 7:43 a.m. ET
But Eli Lilly halts development pact for new version of Prozac antidepressant
By Staff Writer Martha Slud
|
NEW YORK (CNNfn) - Three of the largest U.S. drug makers, Eli Lilly and Co., Bristol-Myers Squibb Co., and American Home Products Corp., posted double-digit increases in third-quarter profits Thursday amid strong sales of their top medicines.
But Eli Lilly, while matching Wall Street's quarterly earnings targets, also disclosed that it has halted a pact with biotechnology firm Sepracor Inc. to develop a new version of Lilly's flagship antidepressant Prozac, raising fresh worries about the company's drug portfolio once Prozac's patent exclusivity expires next year. The company predicted single-digit earnings growth for 2001 because of the expected generic competition to Prozac.
The news triggered a 6 percent drop in Eli Lilly's stock earlier in the session, but the stock recovered later in the day, closing down 75 cents at $88.50. The impact was far greater on Sepracor, a money-losing biotech company that stood to receive millions of dollars in product royalties from a joint product that went on the market. Sepracor shares plummeted nearly 30 percent, falling $33.75 to $87.06.
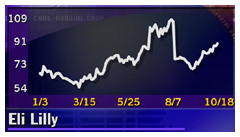
Lilly 3Q on target
Indianapolis-based Eli Lilly (LLY: Research, Estimates) posted a 15 percent increase in third-quarter profit, matching Wall Street forecasts. Net income rose to $778.8 million, or 71 cents per diluted share, up from year-earlier earnings of $679.7 million, or 62 cents per share, excluding a one-time gain from the sale of rights to the antibiotic Lorabid to King Pharmaceuticals. With that gain, year-earlier net income came to $732.6 million, or 67 cents per share.
 The results matched the First Call consensus estimate of analysts. The results matched the First Call consensus estimate of analysts.
Sales rose 9 percent to $2.8 billion from $2.6 billion in the 1999 period. Sales of Prozac, the company's best-selling drug, fell 1 percent to $680.2 million amid heightened competition from other products, while sales of anti-psychotic medication Zyprexa rose 28 percent to $644.9 million and diabetes products sales gained 14 percent to $450 million.
Eli Lilly has been trying to craft new depression treatments amid the expected loss of patent protection for Prozac next year. In August, a U.S. appeals court set a sooner-than-expected end for Lilly's exclusive rights to market Prozac, saying its patent will expire next August.
But the company said Thursday it is dropping a pact with Sepracor Inc. (SEPR: Research, Estimates) to develop the molecule R-fluoxetine as the active ingredient an improved version of Prozac, after receiving disappointing clinical data about the experimental treatment. The company said it decided to concentrate on two other proposed depression medicines instead.
Lilly said it will channel more resources toward the rapid completion of additional trials necessary for the regulatory filing of duloxetine for the treatment of major depression. But the company said it would not be ready to submit an application for that drug to the U.S. Food and Drug Administration until late 2001 or early 2002.
Lilly also said it will continue development of a third depression drug candidate, a combination of the molecules olanzapine and fluoxetine, for "difficult-to-treat" forms of depression.
Eli Lilly has one of the most prolific drug pipelines in the entire pharmaceutical industry, but investors will have to wait several years for it to make up for the expected loss of Prozac patent protection in 2001, said Corey Davis, a drug industry analyst at Chase H&Q in New York.
"They simply cannot make up for the loss of Prozac in late 2001," he said. Chase H&Q has a "market perform" rating on the company's stock.
For the first nine months of the year, Lilly's net earnings rose 18 percent to $2.29 billion, or $2.09 per share. Sales rose 10 percent to $7.89 billion.
Bristol beats by a penny
Bristol-Myers posted a 13 percent increase in quarterly profit. The No. 3 U.S. drug company, which announced last month that it will divest its Clairol hair care and Zimmer orthopedics device units to focus on its drug business, earned $1.24 billion, or 62 cents per diluted share, up from $1.1 billion, or 54 cents per diluted share, in the year-earlier period.
 The results exceeded analysts' forecasts by a penny per share. Analysts polled by earnings tracker First Call had anticipated profit of 61 cents per share. The results exceeded analysts' forecasts by a penny per share. Analysts polled by earnings tracker First Call had anticipated profit of 61 cents per share.
Sales for the quarter rose 5 percent to $5.3 billion. Pharmaceutical sales worldwide rose 12 percent to $3.6 billion, led by a 16 percent gain in sales of cholesterol-lowering treatment Pravachol and 25 percent sales growth for diabetes medicine Glucophage.
For the first nine months, the New York-based company's net earnings rose 14 percent to $3.55 billion, or $1.77 per share, from $3.11 billion, or $1.54 per share, in the 1999 period. Sales rose 7 percent to $15.8 billion.
Bristol-Myers finished the day up 6 cents at $58.94.
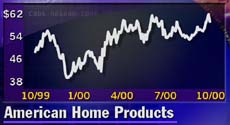
AHP posts 18 percent profit gain
American Home Products Corp. reported an 18 percent increase in third-quarter net earnings, excluding one-time items.
Profit totaled $762.1 million, or 58 cents per diluted share, up from $645.1 million, or 49 cents per share, in the 1999 period. Including a $4.75 billion one-time charge for the company's settlement of the "fen-phen" diet drug litigation, the company posted a net loss of $2.64 billion, or $2.02 per share, in the year-earlier third quarter. The latest results matched the First Call consensus estimate.
Madison, N.J.-based AHP (AHP: Research, Estimates), the maker of estrogen replacement drug Premarin as well as over-the-counter products Advil and Chapstick, posted a 13 percent sales increase to $3.68 billion. Pharmaceutical sales increased 14 percent.
Despite the multibillion-dollar fen-phen settlement earlier this year, the company's diet drug woes are not going away easily. The company reiterated previous statements that additional reserves likely will be required to pay for the ongoing fen-phen litigation. AHP said it will take additional charges to shore up its reserves to pay for future litigation associated with the roughly 45,000 people who declined to participate in the settlement and are pursuing separate court cases.
AHP recalled diet drugs Pondimin and Redux - which made up the "fen" in the diet drug combination -- in 1997 after some of the 6 million Americans who had taken the obesity treatment developed heart problems, including leaky valves. Phentermine, the second half of the diet treatment, still is sold by other companies and has not been linked to the problems.
 Company officials said they could not predict the extent of the additional reserves required. They said only that it will be less than the amount recorded in the 1999 third quarter, meaning the company could still take billions in additional charges related to fen-phen. Part of the charge is expected to be taken in the fourth quarter of 2000. Company officials said they could not predict the extent of the additional reserves required. They said only that it will be less than the amount recorded in the 1999 third quarter, meaning the company could still take billions in additional charges related to fen-phen. Part of the charge is expected to be taken in the fourth quarter of 2000.
For the first nine months, net income totaled $1.45 billion, or $1.10 per diluted share, compared with a loss of $1.82 billion, or $1.39 per share, in the year-earlier period, including special items.
The 2000 results include the divestiture of the company's Cyanamid Agricultural Products unit in the second quarter, as well as a $1.8 billion gain from the termination fee resulting from Warner-Lambert Co.'s aborted merger with AHP. Warner-Lambert merged instead with Pfizer Inc. (PFE: Research, Estimates).
AHP shares fell 88 cents to $59.13. 
|
|
|
|
|
 |

|

