|
Prozac patent extended
|
 |
November 15, 2000: 4:20 p.m. ET
FDA grants Eli Lilly six-month extension for patent on antidepressant
|
NEW YORK (CNNfn) - Eli Lilly & Co. won a six-month patent extension for its blockbuster antidepressant Prozac on Wednesday, meaning that generic rivals could go on the market as early as next August unless the company succeeds in a federal appeal to block copy-cat treatments for several more years.
The U.S. Food and Drug Administration, as expected, granted the so-called "pediatric extension" after Lilly submitted data regarding the effects of Prozac on child depression. The FDA often grants such extensions if drug makers supply information about the use of their treatments in children when there is a lack of existing scientific data.
The extension doesn't mean that the drug is approved for pediatric use, but only that the company complied with the FDA request to supply the data.
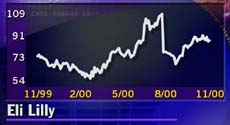
The extension gives Lilly (LLY: Research, Estimates) until Aug. 2, 2001, to retain its patent protection for Prozac. The decision follows an August ruling by a federal appeals court in Washington that found Lilly's Prozac patent would expire in early 2001.
However, Lilly argues that the Prozac patent should stretch through 2003 and has asked for a re-hearing before the appeals court. The company's chief executive officer, Sidney Taurel, told CNNfn last month that Lilly is prepared to fight all the way to the Supreme Court to try to retain exclusive rights to market Prozac for the next three years.
Prozac sales totaled about $2.6 billion last year. The introduction of cheaper, generic rivals is expected to reduce Lilly's earnings growth in 2001 and 2002 to the single digits.
Lilly stock closed down 19 cents at $87.69 at 4 p.m. ET Wednesday on the New York Stock Exchange. 
|
|
|
|
|
|
Eli Lilly
|
Note: Pages will open in a new browser window
External sites are not endorsed by CNNmoney
|
|
|
|
 |

|

