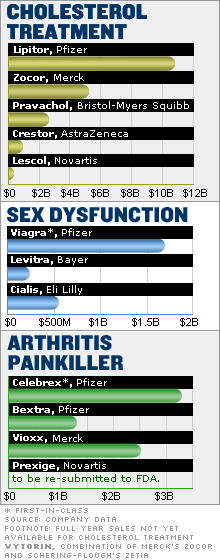
 |
| Correction
|
| An earlier version of the graphic above misidentified the maker of Vioxx. CNN/Money regrets the error.
|
|
|
|
|
|
NEW YORK (CNN/Money) -
Some scientists look down their noses at me-too drugs, but they can't help but notice the smell of money.
Derisively called knock-offs and copycats, me-too drugs follow on the heels of groundbreaking, first-in-class treatments. Drug makers now know that me-too drugs can be just as profitable -- sometimes even more so -- than their innovative predecessors.
"[Me-toos] can be quite lucrative," said David Moskowitz, analyst for Friedman, Billings, Ramsey & Co.
"People who are more science-oriented and innovative tend to look down on me-too drugs," said Al Rauch, analyst for A.G. Edwards & Sons. But "there's a lot more risk in being innovative."
Lipitor, king of the me-toos
Look no further than Lipitor, developed by Pfizer to lower cholesterol. It was the fourth drug of its kind to hit the market. But it did $10.8 billion in 2004 sales and is the top-selling drug of all time.
The first big cholesterol drug in this group was Merck's (up $0.21 to $31.43, Research) Mevacor, which has gone generic and is now known as lovastatin.
Bristol-Myers Squibb (up $0.25 to $25.35, Research) followed with Pravachol, and then Merck again with Zocor. Both of these drugs were blockbusters -- but not like Lipitor, the first drug to reach $10 billion in annual sales.
Analysts say that Lipitor beat its rivals because it was able to work off earlier treatments to make a better drug. It's more effective in lowering low-density lipids and proteins, which transport cholesterol in the blood.
"Me-too drugs [like Lipitor] provide incremental improvements," said Rauch. "Not everything can be completely innovative."
Despite the success of Lipitor and other cholesterol blockers, me-toos don't always prevail over their predecessors. Richard Evans, analyst for Bernstein & Co., Inc., said that me-toos tend to have a "lower risk/return profile than original ideas."
"On average they generate lower sales and margins than innovators, assuming they don't have a major advantage [like Lipitor,]" said Evans. "But that's a reasonable trade for their relatively higher odds of approval and lower costs of development."
For cox-2s, only the first-in-class survived
In the beleaguered class of arthritis pain relievers, known as cox-2 inhibitors, the first to hit the market, Pfizer's Celebrex, is the only one left standing.
Celebrex, which generated $3.3 billion in sales in 2004, has suffered a steep decline in prescriptions this year after the Food and Drug Administration announced it is considering giving Celebrex a "black box" label, the most serious government health warning.
Nonetheless, Celebrex has fared far better than the me-toos, which have all been withdrawn because of health risks.
Pfizer pulled its other cox-2 inhibitor, Bextra, from the market on April 7 at the request of the FDA. A few months before, on Sept. 30, 2004, Merck pulled Vioxx from the market because tests had shown increased risk of heart attacks and strokes.
So how did Celebrex survive? In this case, the me-toos' better efficacy in inhibiting cox-2s might have backfired, said Moskowitz.
"The next generation products [Vioxx and Bextra] were meant to be better, but better meant more side effects and risks," he said.
Bextra and Vioxx were blockbusters before they were pulled from the market. Novartis (up $0.06 to $48.86, Research) is trying to bring another cox-2 inhibitor on the market, having originally filed Prexige with the FDA in 2002. Novartis then pulled its application "based on concerns about the [cox-2 inhibitor] class," said Novartis spokesman Nehl Horton. Novartis plans to re-submit the drug in 2007.
Viagra is still king
Drug makers dream of developing a success like Viagra, an innovative, first-in-class blockbuster that broke new ground and became a household name. With annual sales of $1.7 billion, Pfizer's Viagra is still way ahead of other treatments for erectile dysfunction.
Rauch said the ED sector is different from cox-2 because there is a finite number of patients and a cap on potential sales. Since Viagra was the first ED drug, it "captured all the market," said Rauch.
"The other drugs that came onto the market basically stole Viagra's share," said Rauch. "The smaller the market, the more difficult it is to get another product. If you're the originator, you have a better chance of becoming the standard."
But in the drug industry, change is generally good, and new products are necessary to move the industry forward.
"As potential patients, we would want these companies to make incremental improvements," said Moskowitz, the FBR analyst. "Without it, we would end up only with first-in-class products that are not as good as they could be."
None of the analysts in this story own stock in companies mentioned here, but a family member of Rauch owns Pfizer stock. Bernstein and FBR does not do business with the companies, but A.G. Edwards does business with some of them.
For more stories on Fortune 500 companies, click here.

|