 |
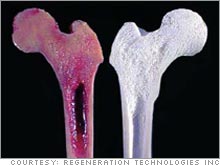
| Regeneration Technologies displays a bone graft that has been BioCleansed (right) versus one that has not (left.) |
|
|
|
NEW YORK (CNN/Money) -
In the 1959 French film "Eyes Without a Face," a doctor kidnaps young women and surgically removes their faces to try and repair his horribly deformed daughter.
In real life, companies are working on something less furtive: allografts, or transplanted tissue taken from corpses. These companies are not transplanting faces, but bones, skin, heart valves and tendons. Companies marketing these products face a slew of challenges, such as keeping the cadaverous parts sterile prior to surgery.
"The gold standard, if you will, is being able to have a label on the product that says it's sterile," said analyst Keay Nakae of financial advisory firm C.E. Unterberg, Towbin.
Regeneration Technologies (down $0.12 to $8.00, Research) and Cryolife (down $0.14 to $6.96, Research) are both poised to take advantage of this growing field and offer a variety of allograft transplant parts. Raj Denhoy, analyst for Piper Jaffray, said the allograft industry totals $900 million. Both analysts said the industry is growing 10 to 15 percent annually.
Regeneration Technologies, a $92 million company based in Alachua, Fla., focuses on heart valves, bone implants and bone paste, which is used to plug holes. The company's allografts include femoral, fibular and tibial "shafts" that are used to buttress breaks, and Achilles tendons that are used in ligament reconstruction for sports injuries.
The demand for tissue grafts outweighs the annual supply of 22,000 donations, said Regeneration Technologies spokeswoman Wendy Crites-Wacker, so the company is branching into the area of xenografts, or tissue grafts from animals. Crites-Wacker said her company received clearance from the FDA to produce some types of bone grafts from cows. In 2006, Regeneration Technologies will use a herd of California beef cattle to produce bone cubes and chips, used to patch holes in bone, as well as bone screws to hold damaged tendons in place.
In addition, Regeneration Technologies uses BioCleanse, a mélange of chemicals, temperature and pressure, to sterilize bones and tendons.
Cryolife, a $62 million company based in the Atlanta suburb of Kennesaw, "cryopreserves" or freeze-dries heart valves, vascular tissue and knee cartilage for transplant, and produces BioGlue, a surgical adhesive. BioGlue is a strong earner for the company, bringing in $39 million in 2004. In addition, Cryolife sterilizes allografts by zapping them with high doses of gamma radiation, using a technology it licenses from Los Angeles biotech Clearant.
The companies obtain their human parts from hundreds of non-profit tissue banks, located around the country. "In the U.S., it's illegal to buy and sell human tissue, so they pay what's called a processing fee," said Denhoy.
Both companies have seen wild volatility in stock prices over the last 12 months. But analysts said the field for allografts is fueled by an aging population in dire need of spare parts.
According to Regenerative Technologies, allografts are used in more than 600,000 surgical procedures in the United States annually. Other options for body part implants include synthetic parts made of metal, and autografts, transplants taken from the patient's own body.
"The advantage to [metal implants] is that they're sterile," said Nakae. "But the disadvantage is they don't remodel over time."
While patients often get good results from autografts, the patients suffer additional surgery because they have to produce their own replacement tissue.
"The allograft remodels like an autograft, but doesn't require second surgery to harvest tissue from the patient, like you do with the autograft," said Nakae.
The need for skin grafts has allowed another company to flourish, LifeCell Corp. (down $0.08 to $21.95, Research), which specializes in AlloDerm. The New Jersey company, which has seen its stock price more than double over the past 12 months, describes AlloDerm as a "dermal matrix" made from donated human skin and freeze-dried for storage. LifeCell produces skin grafts to patch up damage from hernias bad enough to cause intestines to spill out.
"[AlloDerm] is a human-derived material that is far superior to anything that can be made," said Denhoy. "[LifeCell's] a real high flyer and the stock's done really well."
Sterility can make or break an allograft company. After a Minnesota man died after receiving an orthopedic implant processed by Cryolife, the FDA investigated the company in 2001 and 2002 and forced the company to recall unused implants.
Cryolife was hit by lawsuits and its allograft products were pulled off the market. But Cryolife managed to satisfy the FDA's quality requirements and was allowed to re-distribute its products.
"It was never actually proven that the tissue was infected, but the individual who got it died," said Denhoy. "The FDA came down pretty hard on Cryolife. The lawyers saw blood in the water. But the company definitely warrants a fresh look, given that I think they're out of the woods."
To address the wider allograft industry, the FDA established its Good Tissue Practices, a set of industry rules, in 2005. But Denhoy said allografts are still a "gray area" with the FDA and face less regulation than medical devices.
The analysts interviewed for this story do not own stock in the companies mentioned here.
To read about medicinal maggots, leeches and other unusual devices, click here.

|