21 Tylenol Children's meds recalled
Children's and infant's liquid medicines will be pulled from warehouses and stores as a precaution against possible contamination.
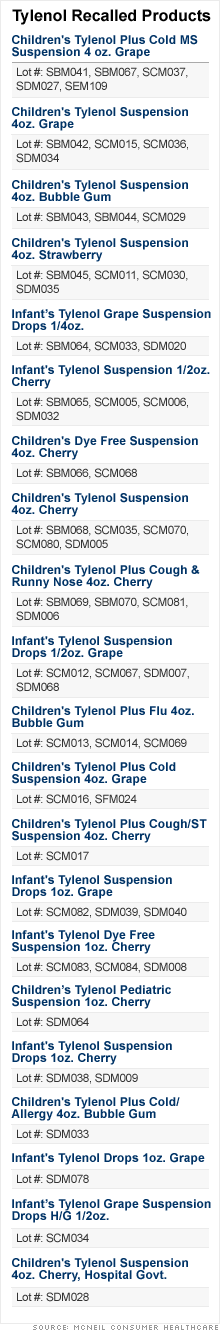
NEW YORK (CNNMoney.com) -- The makers of Tylenol are recalling 21 children's and infant's Tylenol liquid products manufactured between April 2008 and June 2008 from warehouses and retail stores as a safeguard against potential contamination.
In a written statement, McNeil Consumer Healthcare, Tylenol's manufacturer, said it detected bacteria in an inactive ingredient. While that ingredient was not used in any packaged final products, it was produced at the same time as those products.
In consultation with the U.S. Food and Drug Administration, McNeil, a subsidiary of Johnson and Johnson (JNJ, Fortune 500), decided to "recall all product that utilized any of the raw material manufactured at the same time as the raw material that tested positive for the bacteria" as a precaution, adding that "the likelihood of a serious medical event is remote."
According to McNeil's statement, scientific literature about the bacteria suggests that ingesting a contaminated pharmaceutical product orally doesn't trigger an infection, but use of products such as a nasal spray with the bacteria has lead to infections.
"Our stomachs have a lot of enzymes that help destroy the bacteria," said Daryl DePestel, a professor at Univesity of Michgan's College of Pharmacy, adding that most foods have bacteria but are killed inside the body. "Using a nasal spray, you could inhale the bacteria into the lungs, bypassing the body's safety mechanisms."
Though the infection could be severe for patients with lung disease, cystic fibrosis or weak immune systems, DePestel said "the risk is pretty low."
"Generally, this particular organism is considered to have a low pathogenicity," he said. "It's not much of a concern."
McNeil advises concerned parents to contact their health provider.
Tylenol was not immediately available for comment. ![]()

