NEW YORK (CNN/Money) -
Shares of GenVec skyrocketed in early Friday trading after the pharmaceutical company said it has signed a contract amendment to start developing a clinical grade vaccine for the flu-like SARS virus.
GenVec said late Thursday that it has signed an expansion to its existing agreement with the National Health Institute's Vaccine Research Center to begin using GenVec's adenovector technology to develop a clinical grade vaccine against SARS. Under the contract amendment, GenVec will receive $420,000.
The adenovector technology calls for removing disease DNA from an adenovirus -- a virus that causes respiratory disease, including one form of the common cold -- and adding a therapeutic gene to the virus, described by GenVec as "nature's highly efficient delivery vehicle."
Scientists have linked SARS to the coronavirus, also a cause of the common cold. GenVec said in a statement Thursday that it would use the SARS coronavirus genome sequence to develop vaccine candidates from the adenoviral vector.
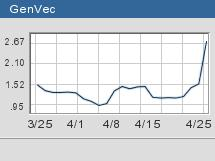
Shares of GenVec (GNVC: up $1.12 to $2.65, Research, Estimates) jumped as much as 96 percent on the announcement.
The World Health Organization has reported 2,422 SARS cases in China and 1,488 in Hong Kong. The United States has 39 "probable" cases of the disease and 208 cases listed as "suspect," according to the U.S. Center for Disease Control.
The WHO said there are about 4,500 cases with 263 deaths worldwide.

|

