|
The war on cancer, 30 years on
Experimental breast cancer drug from Glaxo, products from Bristol and Pfizer on tap for oncology conference.
NEW YORK (CNNMoney.com) - More than 30 years have passed since President Richard Nixon declared a "war on cancer," and while no single cure has been found, many new treatments have come to market. And there are more on the way, with some of the newest drugs taking center stage at the annual conference of the America Society of Clinical Oncology (ASCO), to be held in Atlanta from June 2-6.
One experimental drug to be highlighted targets an aggressive form of breast cancer. British drugmaker GlaxoSmithKline (Research) plans to release results for tykerb, a potential treatment for women with breast cancer that has spread to the brain. The need for breast cancer therapies cannot be understated: About 275,000 women will be diagnosed with breast cancer in the U.S. in 2006 and 40,000 women are expected to die from it this year, according to estimates from the National Breast Cancer Coalition. The Glaxo treatment, a pill, is being tested in women with the aggressive HER2 breast cancer, which is present in about half of all cases. Glaxo spokeswoman Mary Anne Rhyne said tests with tykerb were so successful that the clinical trials were halted early. Her company plans to release data at the conference on patients whose breast cancer did not respond to the drug Herceptin, but then took tykerb in conjunction with a drug from Roche called Xeloda, she said. Tykerb is similar to Herceptin, an injectable drug from Genentech that had sales of about $750 million last year. "We've been investing heavily in oncology research and this is a chance for us to demonstrate progress," said Rhyne, adding the company is conducting more tests to see if tykerb can be used to fight other cancers. Analyst Gbola Amusa at the research firm Sanford C. Bernstein said he expects strong results for tykerb at the conference. "Patients who had failed everything else got better on tykerb," said Amusa. "This drug, if the data is positive at ASCO, will offer unmet medical needs." Amusa projects that the Food and Drug Administration will approve tykerb and that annual sales will reach $1.5 billion by 2012 and could go higher. Amusa said tykerb has potential for many different forms of cancer treatment and that many doctors will prescribe it "off label," meaning they will prescribe it for uses that are not approved by the FDA, which could lead to even more sales. The FDA has tykerb on a "fast track" accelerated review the agency reserves for drugs with life-saving potential. The drug industry for cancer, America's second-biggest killer behind heart disease, is growing rapidly. Cancer drug sales are expected to more than double over five years, from $24 billion in 2004 to $55 billion in 2009, according to IMS Health, the healthcare research firm. More drugs on tap
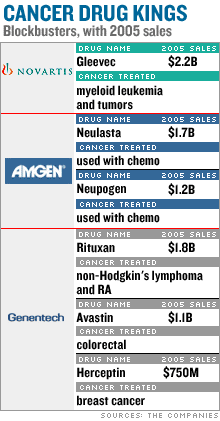
Meanwhile, the nation's two biggest biotechs, Amgen (down $1.27 to $67.10, Research) and Genentech (down $2.85 to $75.93, Research), have emerged as leaders in the market for cancer drugs. Genentech, of South San Francisco, makes the blockbusters Rituxan, a treatment for non-Hodgkin's lymphoma and rheumatoid arthritis with $1.8 billion in 2005 sales, and Avastin, for colorectal cancer with $1.1 billion. Amgen, based in Thousand Oaks, Calif., makes two treatments for chemotherapy patients: Neupogen, a $1.2 billion drug, and Neulasta, at $1.7 billion. Amgen, Genentech, and other companies are also expected to release data on cancer drugs at the conference. For its part, Bristol-Myers Squibb (up $0.02 to $24.15, Research) plans to release data on another experimental cancer drug, dasatinib, which is being tested as a treatment for chronic myeloid leukemia. The FDA could complete its accelerated review of dasatinib as early as June 28, even though the drug has yet to enter the final phase of testing, Bristol spokesman Tony Plohoros said. He said dasatinib was "potentially a pipeline within a [single] product" with a wide range of potential uses, and was being tested as a treatment for cancer of the breast and pancreas, among others. Dasatinib could total $500 million in annual sales, but competition with Novartis' (up $0.21 to $56.81, Research) Gleevec will keep it from becoming a billion-dollar drug, said Les Funtleyder, analyst for Miller Tabak. Patients who take dasatinib will be those who tried Gleevec first but failed to see any improvement, he said. Gleevec sales totaled $2.2 billion in 2005 and the drug is the second-biggest seller for Swiss drug giant Novartis. But like many cancer drugs, Gleevec is expensive for patients, costing about $40,000 annually, said Al Rauch, analyst for A.G. Edwards. Still, the drug has become profitable since it means patients don't have to endure expensive chemotherapy, said Rauch. Pfizer (down $0.38 to $23.89, Research) is expected to release data on Sutent, recently approved by the FDA for stomach and kidney cancer. While sales of cancer drugs are growing briskly, high prices could eventually hinder that due to growing resistance to rising medical costs, said Rauch of A.G. Edwards. "The drug industry has come out and said they're going to try to control the prices," he said, adding that products that aren't unique will also face pressure from competitors. The analysts interviewed for this story do not own shares in the companies mentioned here, though a family member of Rauch owns shares in Pfizer. ----------------------------------- 2 biotechs ready for an upswing. More here.
For more on Merck's cervical cancer vaccine, click here. |
|