|
Drug giants fight for diabetes market
Merck and Novartis testing two similar drugs, both under FDA review and hailed as potential blockbusters.
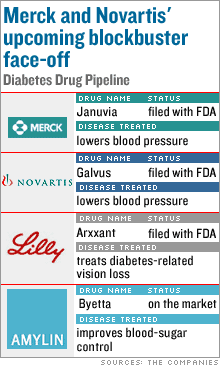
NEW YORK (CNNMoney.com) - The drug giants Merck and Novartis could soon be locking horns for dominance in the multibillion-dollar diabetes sector. Merck (down $0.44 to $34.00, Research), the No. 2 U.S. drug manufacturer, and Novartis (down $0.39 to $56.11, Research), the Swiss drug giant, have similar diabetes drugs in their pipelines and could soon be vying for control in the market. Merck's Januvia and Novartis' Galvus are both being reviewed by the Food and Drug Administration to lower blood sugar in patients with type 2 diabetes, the most common type of the disease.
"It's Januvia versus Galvus, Merck versus Novartis: a face-off," said Les Funtleyder, analyst for Miller Tabak. Both these experimental drugs are in the same drug class, known as DPP-4 inhibitors. Analysts expect these potential blockbusters to slug it out for market share as early as this year, assuming they're both approved by the FDA. "You really have two products battling it out," said Jon LeCroy, analyst for Natexis Bleichroeder. "The question is: who's the winner and who's taking second share?" The companies are expected to unveil clinical trial data about these two drugs at the American Diabetes Association's annual conference June 9-13 in Washington, D.C. The U.S. drug market for diabetes is at least $12 billion and growing, fueled by bad health and obesity, a problem that many health providers call "diabesity." In diabetes, the pancreas fails to produce enough insulin, which converts sugar into energy. The ADA says 20.8 million Americans are diabetic, while another 41 million are at risk. Most of those with "pre-diabetes" health conditions don't even know it. More than 90 percent of diabetics are type 2, which develops as a result of bad health. Most of these cases are preventable and emerge in adults, though more children are getting diagnosed with type 2. The more serious type 1 diabetes, where the pancreas does not produce insulin at all, usually emerges in younger people and is not considered preventable. Merck vs. Novartis
Merck is likely to beat its competitor to this robust market, since it filed its Januvia application to the FDA in February, ahead of Novartis' Galvus filing in March. Merck said the FDA is expected to make its decision on Januvia by mid-October. Even though Merck has a lead in getting its drug to the market, LeCroy said that Novartis will be the likely winner. "Based on data we've seen over the past several years, Galvus looked like it lowered [the measure of blood-sugar] better than Januvia," LeCroy said. LeCroy projects annual sales of $750 million for Januvia by 2010 and said sales would be higher for Galvus, but he did not provide an estimate. Funtleyder of Miller Tabak projected $1 billion in potential sales for Januvia, compared to $1.5 billion for Galvus. Bernstein analysts project $1.5 billion in annual Galvus sales by 2010, rising to $2.1 billion in 2012. Merck is also investigating combining Januvia with metformin, a generic blood-sugar drug, and plans to file this combination, MK-0431A, with the FDA in 2007. If successful, the combination could bring in additional sales for the company. Both drug makers have enjoyed recent successes with their pipelines, which long-term investors watch closely. On May 19, an FDA advisory committee recommended the approval of Gardasil, Merck's vaccine for the virus that causes most cases of cervical cancer. The FDA will make its final decision June 8. On May 17, Novartis released strong data on Rasilez, an experimental drug for hypertension, showing that the drug significantly reduces blood pressure over a 24-hour period. Novartis filed its Rasilez application to the FDA in April. Funtleyder of Miller Tabak projects sales of $1 billion for Rasilez. Other drugs: Arxxant and Byetta
The studies on Januvia and Galvus will spur the most excitement at the ADA conference, analysts say. But in addition, Eli Lilly & Co. (up $0.25 to $50.66, Research) will announce study results for Arxxant, an experimental treatment for diabetes-related vision loss that the company filed with the FDA in February. The FDA has given Arxxant fast-track review status, and it could become the first treatment for this particular type of vision loss. But since the drug failed in separate clinical trials as an effective treatment for nerve disease related to diabetes, sales projections are relatively light. LeCroy estimates annual peak sales of $250 million for Arxxant. Funtleyder said he "wasn't that optimistic" about Arxxant because "it's had a bit of a checkered past in terms of clinical trials." Amylin (up $0.40 to $39.43, Research) and Lilly are also expected to release study results on Byetta, a drug that improves blood-sugar control in diabetics and was launched in the U.S. in 2005. Byetta, which is based on the active compound exenatide, is an injectable drug that is taken twice a day. Amylin, Lilly and Alkermes (down $0.46 to $18.28, Research) are also testing long-acting release (LAR) exenatide, which would be taken once a week. _________________
To read about Exubera, the inhalable insulin, click here. |
|