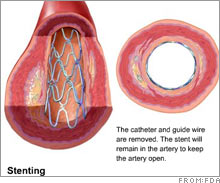
Blood clot risk of device probedGovernment appears to support study showing drug-coated stents increase risk of clotting and could spur heart attacks.NEW YORK (CNNMoney.com) -- An FDA advisory committee will be taking a hard look this week at drug-coated stents, which the agency approved back in 2003 to prevent the arteries from re-closing better than non-drug stents. The review could affect drug-coated stent-makers such as Johnson & Johnson (down $0.18 to $66.10, Charts) and Boston Scientific (up $0.46 to $16.40, Charts). It could also affect Medtronic (down $0.40 to $52.95, Charts), which is awaiting a decision for its drug-coated stent from the Food and Drug Administration.
The stents, which are placed into the arteries of heart attack survivors and high-risk patients, are coated with blood-thinners to prevent clotting that could cause heart attacks. But according to a study from the Cleveland Clinic announced Nov. 29, the drug-bearing mesh surface of these newer stents increased the chance of blood clots by four or five times over the older bare-metal stents. The Ohio-based medical research and education facility conducted 14 studies with 6,675 patients, and concluded that the increased risk of clotting lasts for a long time after the stents are implanted and could trigger a heart attack. An FDA committee plans to review drug-coated stents at meetings on Thursday and Friday. The FDA cited numerous stent studies in documents released Tuesday, and said that some studies "suggested a small but significant increase in death" that is "possibly" be related to drug-coated stents. Dr. Deepak Bhatt of Cornell University Medical College in New York City and senior author of the Cleveland Clinic study, said he wasn't sure why the mesh-surface stents were increasing the risk of blood clots and heart attacks. "No one can say for sure what the increased risk is due to, but it might be that the drug-coated stents are less likely than bare-metal stents to get covered with the body's own layer of cells," wrote Dr. Bhatt in an e-mail to CNNMoney.com. "This may then predispose the exposed stent struts to flowing blood and a clot may form. Likely, anti-clotting drugs would minimize this risk, though that is not entirely proved." The American Heart Association released a statement Tuesday saying there was "conflicting data regarding the magnitude and significance" of the blood clot risk of mesh stents - as compared to bare metal stents - that possibly emerges one year after the stents have been implanted. "Some of the patients in these longer-term studies were no longer taking aspirin, which is usually indicated on a lifelong basis for all patients with known coronary heart risk," said the AHA statement. "Additional studies will probably be required to define the risk of late blood clots in patients with drug-eluding stents and the appropriate therapy to prevent them." Tao Levy, analyst for Deutsche Bank, said the FDA's pre-meeting documents contained "no major surprises" and were "not onerous" for J&J, Boston Scientific and Medtronic, which he has given "buy" ratings. "It does not appear that the FDA is prepared to take [drug-coated stents] off the market and more likely will adjust the label," wrote Levy, in a published report. Levy downplayed the significance of a stricter warning label, noting that most patients who get drug-coated stents don't always fit the drugmakers' profile of the patients for whom the stents were intended. Deutsche Bank makes a market in the companies mentioned in this story. |
|