|
'Morning-after' pill wins key FDA approval Barr Pharmaceuticals says regulators approve non-prescription sale of Plan B emergency contraception. NEW YORK (CNNMoney.com) -- Barr Pharmaceuticals said Thursday the Food and Drug Administration approved the over-the-counter sale of its "morning-after" pill. The approval gives women 18 years and older non-prescription access to the Plan B emergency contraception pill. Women 17 years and younger still will need a prescription, the company said.
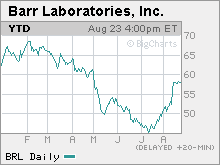
Plan B is made by Duramed Pharmaceuticals Inc., a subsidiary of Barr Pharmaceuticals. Shares of Barr Pharmaceuticals (Charts) rose 1.2 percent in early Wall Street trading on the approval. "While we still feel that Plan B should be available to a broader age group without a prescription, we are pleased that the Agency has determined that Plan B is safe and effective for use by those 18 years of age and older as an over-the-counter product," Barr CEO Bruce Downey said a statement. Plan B, an emergency oral contraceptive that can prevent pregnancy if taken within 72 hours after intercourse, has been sold as a prescription drug since FDA approval in 1999. But it has faced difficulties winning approval for over-the-counter sales. In 2003, the FDA's advisory panel voted 24 to 3 in favor of OTC status, but the agency did not follow the advice of its panel, as it usually does, and later denied OTC status. |
|