Heart drug pulled, Pfizer tumblesWorld's biggest drugmaker halts trial after results show drug caused an increase in deaths, heart problems.NEW YORK (CNNMoney.com) -- Pfizer stock tumbled Monday after the world's biggest drugmaker abruptly pulled the plug on its most important experimental medicine - a drug meant to treat heart disease that instead caused an increase in deaths and heart problems in people taking it in a clinical trial. Shares of Pfizer (Charts) sank about 11 percent in afternoon trading as investors worried what the New York-based company would do to replace the product, torcetrapib, in its pipeline.
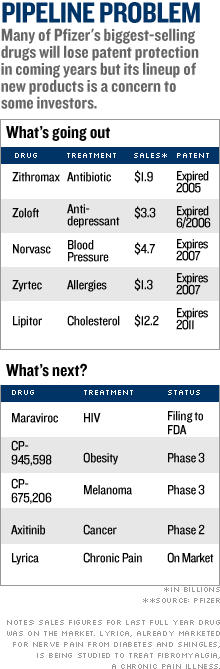
Trading was heavy with more than 235 million shares changing hands by mid-afternoon - nearly seven times the stock's average daily volume. On Saturday, Pfizer and the Food and Drug Administration announced that the drug company would halt a clinical trial of torcetrapib due to an increased rate of death and heart problems in patients who took it. Just two days earlier, Pfizer's new CEO Jeffrey Kindler had told hundreds of investors and analysts at a research meeting that the drugmaker could seek approval for the medicine as early as next year if clinical data supported it. Pfizer had planned to pair torcetrapib with Lipitor, the world's best-selling drug that cuts bad cholesterol, to maintain sales once Lipitor loses U.S. patent protection in 2011. The patients were taking torcetrapib to boost so-called "good" cholesterol, along with Lipitor. Lipitor alone was not associated with an increased rate of death in the clinical trial. The news was a setback for the millions of people looking to control their cholesterol to battle heart disease, which kills more than 650,000 Americans a year, making it the leading cause of death in the United States. Dr. Daniel Fisher, cardiologist at NYU Medical Center and clinical assistant professor at NYU School of Medicine, said the failure of the torcetrapib trial was a "big blow" to heart disease treatment. "This throws a big wrench in the system," said Dr. Fisher. "[Torcetrapib] would have been a very big thing. We're sort of stuck with what we've got, for now." The abrupt halt to the trial ends Pfizer's biggest drug study, one on which it spent about $800 million on research involving 25,000 patients at hundreds of locations around the world. The sudden end to the trial was a major setback for Pfizer, which is already cutting jobs as it struggles with replacing a valuable stable of drugs that are going off patent in the next few years. "Torcetrapib torpedoed," wrote Miller Tabak analyst Les Funtleyder in a note to clients. "Obvious question [is] what next? Our view is more cost-cutting and a few acquisitions on the horizon." Analyst Barbara Ryan at Deutsche Bank North America cut her price target on the stock to $28 from $33 but kept a "buy" rating on the stock. Pfizer said just last week that it would cut more than 2,000 sales reps from its 11,000-strong U.S. sales force. Lipitor, the world's top-selling drug with sales of $12.2 billion in 2005, will lose patent protection in 2011, opening up the drug to generic competition. Pfizer executives were hoping torcetrapib would help fill the hole left after Lipitor goes off patent. They had created a patented Lipitor combo drug with torcetrapib. But now that late-stage tests have been stopped, Pfizer will have to rely on other potential drugs in its pipeline. This includes the HIV drug Maraviroc, which has been filed with the FDA, and late-stage experimental treatments for cancer and obesity. Chris Schott, an analyst for Bank of America, wrote in a published note that Pfizer also could start looking harder to buy companies with strong pipelines. The analyst said that despite the big setback with torcetrapib, he was keeping a "buy" rating for Pfizer, noting he believes its pipeline is "underappreciated by the market." Pfizer, like the No. 4 U.S. drugmaker Merck, has been buying up smaller companies with promising pipelines and licensing late-stage drugs, in addition to developing its own drugs in-house. But Ryan of Deutsche Bank North America said Pfizer needs to "step up the size and scale" of its acquisitions, to quickly fill the vacuum left by torcetrapib. Pfizer needs to continue to slash costs, said Ryan, and should increase its dividend so shareholders won't jettison the stock. "A dividend increase of 20 percent to 25 percent next week is warranted and now inevitable," wrote Ryan in a published note. Despite Pfizer's woes, other drug stocks were mostly higher. Merck (Charts), Schering-Plough (Charts) and Bristol-Myers Squibb (Charts) all gained, though Novartis (Charts) shares fell in morning trading. Bank of America does and seeks business with companies covered in its reports, including Pfizer. |
|