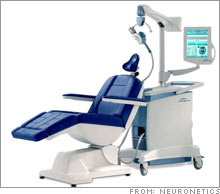
FDA to consider depression-fighting machineNeuroStar machine could compete with antidepressant drug industry, if it wins FDA approval.NEW YORK (CNNMoney.com) -- Millions of Americans have gotten used to popping pills for depression, but the antidepressant of the future might be a machine that pulses magnetic waves through the brain. FDA advisers on Friday will scrutinize the experimental NeuroStar System from the privately-held Pennsylvania company Neuronetics. The NeuroStar, which has not yet entered the market, is designed to treat major depression by pulsing magnetic waves through the mood circuits of a patient's brain.
The advisers will vote on whether the NeuroStar is safe and effective for the U.S. market. The FDA will take this non-binding vote into consideration later this year, when it decides whether to approve the system. The vote is important, because the FDA follows the advice of its advisers most of the time. Instead of taking drugs, the patient sits in a chair and lets the NeuroStar machine do its work, submitting to 40-minute sessions during a period of three to six weeks. The system is designed to treat a form of depression that is too severe for psychotherapy or antidepressants. Peter Anastasiou, vice president of marketing for Neuronetics, said he expects the FDA to make its final decision on the machine in the first half of 2007. If the agency gives the device a green light, Anastasiou said the NeuroStar will be introduced first to "limited markets", focusing on major cities. Competing for acceptance "We believe this type of technology to be the absolute wave of the future," said Stephen Brozak, analyst for WBB Securities, who believes the NeuroStar has blockbuster potential as a billion-dollar-a-year product. But Brozak also said that there isn't enough information to determine whether NeuroStar and its potential competitor, the VNS Therapy System from Houston-based Cyberonics (down $0.11 to $20.19, Charts), are effective enough to win the confidence of doctors and take over the antidepressant market. The VNS is a device that's surgically implanted in the chest and neck. The device treats depression by sending mild shocks to a nerve that serves as a major communication pathway to the brain. FDA advisers are also scrutinizing the VNS system on Friday, focusing on post-market studies of the device. The VNS system has been on the market for years, primarily as a treatment for epilepsy, though since 2005 it has been used to treat major depression. Brozak said that acceptance of the NeuroStar could squeeze into Cyberonics sales, which gleaned $120 million from the VNS system last year, mostly from epilepsy treatment. Brozak said the NeuroStar machine appears to be less "invasive" than the surgically implanted VNS system. "The Neuronetics device would directly compete with the Cyberonics device if approved," said Brozak. "Just the nature of the fact that the Neuronetics is non-invasive could create problems for Cyberonics." The introduction of the new technology might be a hard sell to physicians, patients and insurers, said Brozak, because doctors are reluctant to take chances with severely depressed patients. But if the machines catch on and gain wider acceptance, they could take a bite of the $12.5 billion industry for antidepressant drugs. The antidepressant industry is currently dominated by Wyeth's (down $0.43 to $50.98, Charts) Effexor, Eli Lilly & Co.'s (down $0.43 to $52.70, Charts) Prozac (which lost its patent in 2001) and Cymbalta, Forest Laboratories' (down $0.71 to $55.38, Charts) Lexapro, GlaxoSmithKline's (down $0.30 to $54.55, Charts) Paxil and Wellbutrin, and Pfizer's (down $0.10 to $26.26, Charts) Zoloft (which lost patent protection in 2006). Brozak does not own stock in Cyberonics and WBB does not conduct investment business with the company. |
Sponsors
|