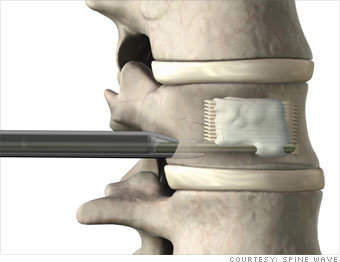
This year Spine Wave received FDA approval for three spinal-implant devices, the StaXx FX, a spinal implant that supports fractures; the StaXx XD, a vertebral replacement used to supplement missing back bones; and the CapSure PS, a screw-based implant that prevents spinal degeneration. U.S. hospitals have purchased the implants, and about 20 physicians are using them in surgery.
Spine Wave plans to greatly increase the distribution of its products in 2008, so it hired a VP of sales to help manage the commercialization of the implants. The company also raised $45 million during its most recent round of financing, for a total of $130 million from its four rounds.
| Sir Richard will help fund U.S. entrepreneurs, jetpacks will finally fly, and GPS devices will show the cheapest gas nearby - plus much more! (more) Watch out, Detroit. A new crop of electric-vehicle startups aims to put a dent in the Big Three by applying the latest in high-technology engineering and design. (more) Behind the scenes at the Boston launch of Virgin Money, Richard Branson's new peer-to-peer lending service. (more) |